Chapter 3: The Cellular Level of Organization
What is cell theory?
The Cell
-Performs all life functions
*PIC*
Sex Cells
-Sex Cells (germ cells):
-Reproductive Cells
-Male Sperm
-Female Oocytes (eggs)
Somatic Cells
-Somatic Cells (Soma=body):
-All body cells except sex cells
Organelle functions
*PIC*
*PIC*
Functions of Cell Membrane
-Physical Isolation
-Barrier
-Regulates exchange with environment
-ions and nutrients center
-waste and cellular products released
-Monitors the environment
-the extracellular fluid composition
-chemical signals
-Structural support
-anchors cells and tissues
What are the structures and functions of the cell membrane?
The Cell Membrane
-contains lipids, carbohydrates, and functional proteins
*PIC*
Phospholipid Bilayer
-Double layer of phospholipid molecules:
-hydrophilic heads: toward watery environment, both sides
-hydrophobic fatty acid tails-inside membrane
-barrier to ions and water soluble compounds
Membrane Proteins:
-Integral proteins:
-within the membrane
-Peripheral proteins:
-inner or outer surface of the membrane
6 functions of Membrane Proteins
1. Anchoring proteins (stabilizers):
-attach to inside or outside structures
2. Recognition proteins (identifiers):
-label cells normal or abnormal
3. Enzymes:
-catalyze reactions
4. Receptor proteins:
-bind and respond to ligands (ions, hormones)
5. Carrier Proteins
-transport specific solutes through membrane
6. Channels:
-regulate water flow and solutes through membrane
Membrane Carbohydrates
-Proteoglycans, glycoproteins, and glycolipids:
-extend cell membrane
-form sticky "sugar coat" (glycocalyx)
Functions of Membrane Carbohydrates
-Lubrication and protection
-Anchoring and locomotion
-Specificity in binding (receptors)
-Recognition (immune response)
Cytoplasm
-All materials inside the cell and outside the nucleus
-cytosol (fluid):
-dissolved materials:
-nutrients, ions, proteins, and waste proteins
-organelles:
-structures with specific functions
What are cell organelles and their functions?
Types of Organelles
-Nonmembranous organelles:
-no membrane
-direct contact with cytosol
-Membraneous organelles:
-covered with plasma membrane
-isolated from cytosol
Nonmembranous Organelles
-6 types od nonmembranous organelles:
-cytoskeleton
-microvilli
-centrioles
-cilia
-ribosomes
-proteasomes
The Cytoskeleton
-Structural proteins for shape ans strength
*PIC*
Microfilaments
-Thin filaments composed of the protein actin:
-provide additional mechanical strength
-interact with proteins for consistency
-pairs with thick filaments of myosin for muscle movement
Intermediate Filaments
-Mid-sized between microfilaments and thick filaments:
-durable (collagen)
-strengthen cell and maintain shape
-stabilize organelles
-stabilize cell position
Microtubules
-Large, hollow tubes of tubulin protein:
-attach to centrosome
-strengthen cell and anchor organelles
-change cell shape
-move vesicles within cell (kinesin and dynein)
-form spindle apparatus
Microvilli
-Increase surface area absorption
-Attach to cytoskeleton
*PIC*
Centrioles in the Centrosome
-Centrioles form spindle apparatus during cell division
-Centrosome: cytoplasm surrounding centriole
*PIC*
Cilia Power
-Cilia move fluids across the cell surface
*PIC*
Ribosomes
-Build polypeptides in protein synthesis
-Two types:
-free ribosomes in cytoplasm:
-proteins for cell
-fixed ribosomes attached to ER:
-proteins for secretion
Proteasomes
-Contain enzymes (proteases)
-Disassemble damaged proteins for recycling
Membranous Organelles
-5 types of membranous organelles:
-endoplasmic reticulum (ER)
-Golgi apparatus
-lysosomes
-peroxisomes
-mitochondria
Endoplasmic Reticulum (ER)
-endo=within,plasm=cytoplasm,reticulum=network
-cisternae are storage chambers within membranes
*PIC*
Functions of ER
-Synthesis of proteins, carbohydrates, and lipids
-storage of synthesized molecules and materials
-transport of material within the ER
-Detoxification of drugs or toxins
Smooth Endoplasmic Reticulum (SER)
-No ribosomes attached
-Synthesizes lipids and carbohydrates:
-phospholipids and cholesterol (membranes)
-steroid hormones (reproductive systems)
-glycerides (storage in liver and fat cells)
-glycogen (storage in muscles)
Rough Endoplasmic Reticulum (RER)
-Surface covered with ribosomes:
-active in protein and glycoprotein synthesis
-folds polypeptides protein structures
-encloses products in transport vesicles
Golgi Apparatus
-Vesicles enter forming face and exit maturing face
*PIC*
Vesicles of the Golgi Apparatus
-Secretory Vesicles:
-modify and package products for exocytosis
-Membrane renewal vesicles:
-add or remove membrane components
-Lysosomes:
-carry enzymes to cytosol
Transport vesicles
-carry materials to and from the golgi apparatus
*PIC*
Lysosomes
-Power enzymes, containing vesicles:
-lyso=dissolve, soma=body
*PIC*
Lysosome Structures
-Primary lysosome:
-formed by Golgi and inactive enzymes
-Secondary lysosome:
-lysosome fused with damaged organelle
-digestive enzymes activated
-toxic chemicals isolated
Lysosome Functions
-Clean up inside cells:
-break down large molecules
-attack bacteria
-recycle damaged organelles
-ejects wastes by exocytosis
Autolysis
-Self destruction of damaged cells:
-auto=self lysis=breakdown
-lysosome membranes break down
-digestive enzymes released
-cell decomposes
-cellular materials recycle
Peroxisomes
-Are enzyme containing vesicles:
-break down fatty acids, organic compounds
-produce hydrogen peroxide (H2O2)
-replicate by division
Membrane Flow
-A continuous exchange of membrane parts by vesicles:
-all memranous organelles (except mitochondria)
-allows adaption and change
KEY CONCEPT:
-Cells: basic structural and functional units of life
-respond to their environment
-maintain homeostasis at the cellular level
-modify structure and function over time
Mitochondrion Structure
-Have sooth outer membrane and folded inner membrane (cristae)
-Matrix
-fluid around cristae
*PIC*
Mitochondrial Function
-Mitochonrion takes chemical energy from food (glucose):
-produces energy molecule ATP
*PIC*
Aerobic Cellular Respiration
-Aerobic metabolism (cellular respiration):
-miochondria use oxygen to break down food and produce ATP
The Reactions
glucose + oxygen +ADP
carbon dioxide + water + ATP
-Glycolysis:
-glucose to pyruvic acid (in cytosol)
-Tricarboxylic acid cycle (TCA cycle):
-pyruvic acid to CO2 (in matrix)
KEY CONCEPT:
-Mitochondria provide cells with energy for life:
-require oxygen and organic substrates
-generate carbon dioxide and ATP
How does the nucleus control the cell?
The Nucleus
-Is the cell's control center
*PIC*
Structure of the Nucleus
-Nucleus:
-largest organelle
-Nuclear envelope:
-double membrane around the nucleus
-Perinuclear space:
-between 2 layers of nuclear envelope
-Nuclear Pores:
-communication passages
Within the Nucleus
-DNA:
-all the information to build and run organisms
-Nucleoplasm:
-fluid containing ions, enzymes, nucleotides, and some RNA
-Nuclear matrix:
-support filaments
Nucleoli in Nucleus
-Are related to protein production
-Are made of RNA, enzymes, and histones
-Sythesize rRNA and ribosomal subunits
Organization of DNA
-Nucleosomes:
-DNA coiled around histones
-Chromatin:
-loosely coiled DNA (cells not dividing)
-Chromosomes:
-tightly coiled DNA (cells dividing)
*PIC*
What is genetic code?
DNA and Genes
-DNA:
-instructions for every protein in the body
-Gene:
-DNA instructions for 1 protein
Genetic Codes
-The chemical language of DNA instructions:
-sequence of bases (A,T,C,G)
-triplet code:
-3 bases= 1 amino acid
KEY CONCEPT:
-The nucleus contains chromosomes
-Chromosomes contain DNA
-DNA stores genetic instructions for proteins
-Proteins determine cell structure and function
How do DNA instructions become proteins?
Protein Synthesis
-Transcription:
-copies instruction from DNA to mRNA (in nucleus)
-Translation:
-ribosome reads code from mRNA (in cytoplasm)
-assembles amino acids into polypeptide chain
-Processing:
-by RER and Golgi apparatus produces protein
mRNA Transcription
-A gene is transcribed to mRNA in 3 steps:
-gene activation
-DNA to mRNA
- RNA processing
Step 1: Gene Activation
-Uncoils DNA, removes histones
-Start (promoter) and stop codes on DNA mark location of gene:
-coding strand is code for protein
-template strand used by RNA polymerase molecule
Step 2: DNA to mRNA
-Enzyme RNA polymerase transcribes DNA:
-binds to promoter (start) sequence
-reads DNA code for gene
-binds nucleotides to form messenger RNA (mRNA)
-mRNA duplicates DNA coding strand, uracil replaces thymine
Step 3: RNA Processing
-At stop signal, mRNA detaches from DNA molecule:
-code is edited (RNA processing)
-unnecessary codes (introns) removed
-good codes (exons) spliced together
-triplet of 3 nucleotides (codon) represents one amino acid
Codons
*PIC*
Translation
-mRNA moves:
-from the nucleus
-through a nuclear pore
*PIC*
-mRNA moves:
-to a ribosome in cytoplasm
-surrounded by amino acids
*PIC*
-mRNA binds to ribosomal subunits
-tRNA delievers amino acids to mRNA
*PIC*
-tRNA anticodon binds to mRNA codon
-1 mRNA codon translates to 1 amino acid
*PIC*
-Enzymes join amino acids with peptide bonds
-Polypeptide chain has specific sequence of amino acids
*PIC*
-At stop codon, components seperate
*PIC*
Nucleus Controls Cell Structure and Function
-Direct control through synthesis of:
-structural proteins
-secretions (environmental response)
-Indirect control over metabolism through enzymes
KEY CONEPT:
-Genes:
-a functional units of DNA
-contain instructions for 1 or more proteins
-Protein synthesis requires:
-several enzymes
-ribosomes
-3 types of RNA
-Mutation is a change in the nucleotide sequence of a gene:
-can change gene function
-Causes:
-exposure to chemicals
-exposure to radiation
-mistakes during DNA replication
How do things get in and out of cells?
Overcoming the Cell Barrier
-The cell membrane is a barrier but:
-nutrients must get in
-products and wastes must get out
Permeability
-Permeability detemines what moves in and out of a cell:
-A membrane that:
-lets nothing in or out if impermeable
-lets anything pass is freely permeable
-restricts movement is selectively permeable
Selective Permeability
-Cell membrane is selectively permeable:
-allows some materials to move freely
-restricts other materials
Restricted Materials
-Selective permeability restricts materials based on:
-size
-electrical charge
-molecular shape
-lipid solubility
Transport
-Transport through a cell membrane can be:
-active (requiring energy and ATP)
-passive (no energy required)
3 Categories of Transport
-Diffusion (passive)
-Carrier-mediated transport (passive or active)
-Vesicular transport (active)
Solutions
-All molecules are constantly in motion
-Molecules in solution move randomly
-Random motion causes mixing
Concentration Gradient
-Concentration is the amount of solute in a solvent
-Concentration gradient:
-more solute in 1 part of a solvent than another
Function of Concentration Gradient
-Diffusion:
-molecules mix randomly
-solute spreads through solvent
-eliminates concentration gradient
Diffusion
-Solutes move down a concentration gradient
Factors Affecting Diffusion Rates
-Distance the particle has to move
-Molecule size:
-smaller is faster
-Temperature:
-more heat, faster motion
-Gradient SIze:
-the difference between high and low concentration
-Electrical forces:
-opposites attract, like charges repel
Diffusion and the Cell membrane
-Diffusion can be simple or channel- mediated
*PIC*
Simple Diffusion
-Materials which diffuse through cell membrane:
-lipid soluble compounds (alchols, fatty acids, steroids)
-dissolved gases (oxygen and carbon dioxide)
Channel-Mediated Diffusion
-Materials which pass through transmembrane proteins (channels):
-are water soluble compounds
-are ions
Factors in Channel-Mediated Diffusion
-Passage depends on:
-size
-charge
- interaction with the channel
Osmosis
-Osmosis is the diffusion of water across the cell membrane
*PIC*
How Osmosis Works
-More solute molecules, lower concentration of water molecules
-Membrane must be freely permeable to water, selectively permeable to solutes
Osmosis Water Movement
-Water molecules diffuse across membrane toward solution with more solutes
-Volume increases on the side with more solutes
Osmotic Pressure
-Is the force of a concentration gradient of water
-Equals the force (hydrostatic pressure) needed to block osmosis
Tonicity
-The osmotic effect of a solute on a cell:
-2 fluids may have equal osmolarity, but different tonicity
Isotonic Solutions
-A solution that does not cause osomotic flow of water in ot out of a cell
-iso=same, tonos= tension
Hypotonic Solutions
-hypo=below
-Has less solutes
-Loses water through osmosis
Cells and Hypotonic Solutions
-A cell in a hypotonic
solution:
-gains water
-ruptures (hemolysis of red blood cells)
Hypertonic Solutions
-hyper=above
-has more solutes
-gains water by osmosis
Cells and Hypertonic Solutions
A cell in a hypertonic solution:
-loses water
-shrinks (crenation of red blood cells)
KEY CONCEPT:
-concentration gradients tend to even out
-In the absence of membrane, diffusion eliminates concentration gradients
-When different solute concentrations exist on either side of a selectively permeable membrane, osmosis moves water through the membrane to equalize the concentration gradients
What are special transport mechanisms
Carrier-Mediated Transport
-Carrier mediated transport of ions and organic substrates:
-facilitated diffusion
-active transport
Characteristics of Carrier-Mediated Transport
-Specificity:
-1 transport protein 1 set of substrates
-Saturation limits:
-rate depends on transport proteins, not substrate
-Regulation:
-cofactors such as hormones
Cotransport
-2 substances move in the same direction at the same time
Countertransport
-1 substance moves in while another moves out
Facilitated Diffusion
-passive
-carrier mediated
*PIC*
How facilitated Diffusion Works
-Carrier proteins transport molecules too large to fit through channel proteins (glucose, amino acids):
-molecule binds to receptor site on carrier protein
-protein changes shape, molecules pass through
-receptor site is specific to certain molecules
Active Transport
-Active transport proteins:
-move substrates against concentration gradient
-require energy, such as ATP
-ion pumps move ions (Na+, K+, Ca+,Mg2+)
-exchange pump contertransports 2 ions at the same time
Sodium-Potassium Exhcange Pump
-Active Transport, carrier mediated:
-sodium ions (Na+) out, potassium ions (K+) in
-1 ATP moves 3 Na+
*PIC*
Secondary Active Transport
-Na+ concentration gradient drives glucose transport
-ATP energy pumps Na+ back out
*PIC*
Transport Vesicles
-Also called bulk transport
-Vesicles:
-endocytosis (endo=into)
-active transport using ATP:
-receptor-mediated
-pinocytosis
-phagocytosis
-exocytosis (exo= out of)
Receptor-Mediated Endocytosis
-Receptors (glycoproteins) bind target molecules (ligands)
-Coated vesicle (endosome) carries ligands and receptors into the cell
*PIC*
Pinocytosis
-Pinocytosis (cell drinking)
-Endosomes "drink" extracellular fluid
*PIC*
Phagocytosis
-Phagocytosis (cell eating)
-pseudopodia (psuedo=false, podia= feet)
-engulf large objects in phagosomes
*PIC*
Exocytosis
-Is the reverse of endocytosis
*PIC*
Summary
-The 7 methods of transport
*PIC*
What is transmembrane potential?
Electrical Charge
-Inside cell membrane is slightly negative, outside is slighty positive
-Unequal charge across the cell membrane is transmembrane potential
-Resting potential ranges from -10 mV to -100 mV, depending on cell type
How do cells reproduce?
Cell Life Cycle
Most of a cell's is spent in a nondividing state (interphase)
*PIC*
3 Stages of Cell Divison
-Body (somatic) cells divide in 3 stages:
-DNA replication duplicates genetic material exactly
-Mitosis divdes the genetic material equally
-Cytokinesis divides cytoplasm and organelles into 2 daughter cells
Interphase
-The nondividing period:
-G zero phase: specialized cell functions only
-G1 phase: cell growth, organelle duplication, protein synthesis
-S phase: DNA replication and histone synthesis
-G2 phase: finishes protein synthesis and centriole replication
DNA Replication
-DNA strands unwind
-DNA polymerase attaches complementrary nucleotides
*PIC*
Mitosis
-Mitosis divides duplicated DNA into 2 sets of chromosomes:
-DNA coils tightly into chromatids
-chromatids connect at a centromere
-protein complex around centremere is kinetochore
Stage 1: Prophase
*PIC*
Features of Prophase
-Nucleoli disappear
-Centriole pairs move into cell poles
-Microtubules (spindle fibers) extend between centriole pairs
-Nuclear envelope disappears
-Spindle fibers attach to kinetochore
Stage 2: Metaphase
*PIC*
Features of Metaphase:
-Chromosomes align in a central plane (metaphase plate)
Stage 3: Anaphase
*PIC*
Features of Anaphase
-Microtubules pull chromosomes apart
-Daughter chromosmes groups near centrioles
Stage 4: Telophase
*PIC*
Features of Telophase
-Nuclear membranes reform
-Chromosomes uncoil
-Nucleoli reappear
-Cell has 2 complete nuclei
KEY CONCEPT:
-Mitosis duplicates chromosomes in the nucleus for cell division
Stage 4: Cytokinesis
-Division of the cytoplasm
*PIC*
Features of Cytokinesis
- Cleavage furrow around metaphase plate
-Membrane closes, producing daughter cells
What regulates cell division?
Miotic Rate and Energy
-Rate of cell division:
-slower miotic rate means longer cell life
-cell division requires energy (ATP)
Long Life, Short Life
-Muscles cells, neurons rarely divide
- Exposed cells (skin and digestive tract) live only days or hours
Chemicals Controlling Cell Division
*PIC*
Regulating Cell Life
-Normally, cell division calances cell lose
Factors Increase Cell Division
-Increase cell division:
-internal factors (MPF)
-extracelluar chemical factors (growth factors)
Factors Decrease Cell Division
-Decreases cell division:
-repressor genes (faulty repressors cause cancers)
-worn out telomeres (terminal DNA segments)
Cancer
*PIC*
Cancer Stages
-Cancer develops in steps:
-abnormal cell
-primary tumor
-metastasis
-secondary tumor
Cell Division and Tumors
-Tumors (neoplasm):
-enlarged mass of cells
-abnormal cell growth and division
Benign Tumors
-Benign tumor:
-contained
-not life threatening
Malignant Tumors
-Malignant tumor:
-spread into surrounding tissues (invasion)
-start new tumors (metastasis)
Cancer and Cells
-Cancer:
-illness that disrupts cellular controls
-produces malignant cells
Cancer and Genes
-Oncogenes:
-mutated genes that cause cancer
KEY CONCEPT:
-Mutations disrupt normal controls over cell growth and division
-Cancers often begin where stems cell are dividing rapidly
-More chromosome copies mean greater chance of erroe
What makes cells different?
Cell Diversity
-All cells carry complete DNA instructions for all body functions
Cell Differentiation
-Cells specialize or differentiate:
-to form tissues (liver cells, fat cells, and neurons)
-by turning off all genes not needed by that cell
KEY CONCEPT:
-All body cells except sex cels, contain the same 46 chromosomes
-Differntiation depends on which genes are active and whcih are inactive
Summary
-Structures and functions of human cells
-Structures and functions of membranous and nonmenbranous organelles
-ATP, Mitochondria, and the process of aerobic cellular respiration
-Structures and functions of the nucleus:
-control functions of nucleic acids
-structures and replication of DNA
-DNA and RNA in protein synthesis
-Structures and chemical activities of the cell membreane:
-diffusion and osmosis
-active transport proteins
-vesicles in endocytosis and exocytosis
-electrical propertes of plasma membrane
-Stages and processes of cell division:
-DNA replication
-mitosis
-cytokinesis
-Links between cell division, energy use, and cancer
Friday, August 27, 2010
Thursday, August 26, 2010
Chapter 2 Notes
Chapter 2: The Chemical Level of Organization
How do atoms work?
KEY CONCEPT:
-Matter is made up of atoms
-Atoms join together to form chemicals with different characteristics
-Chemical characteristics determine physiology at the molecular and cellular level
The Atom
Atomic Particles
-Proton:
-positive, 1 mass unit
-Neutron:
-neutral, 1 mass unit
-Electron:
-negative, low mass
Particles and Mass
-Atomic Number:
-Number of protons
-Mass Number:
-Number of protons plus neutrons
-Atomic Weight:
-exact mass of all particles (daltons)
Isotopes
-2 or more elements with equal numbers of protons but different numbers of neutrons
Elements in the Human Body
*PIC*
-Are determined by the atomic number of an atom:
-are the most basic chemicals
Chemical Properties of Atoms
How do atoms forn molecules and compounds?
Chemical Bonds
-Ionic bonds:
-attraction between cations and anions
-Covalent bonds:
-strong electron bonds
-Hydrogen bonds:
-weak polar bonds
Molecules and Compounds:
-Molecules
-atoms joined by strong bonds
-Compunds
-atoms joined by strong or weak bonds
Ionic Bonds
*PIC*
-Are atoms with positive or negative charge
*PIC*
Covalent Bonds
Hydrogen Bonds
-Hydrogen bonds between H2O molecules cause surface tension
*PIC*
States of Matter
-Solid
-constand volume and shape
-Liquid
-constant volume but change shape
-Gas
-change volume and shape
Why are chemical reactions important to physiology?
Energy
-Energy
-the power to do work
-Work
-a change in mass or distance
Forms of Energy
-Kinetic energy
-energy of motion
-Potential Energy
-stored energy
-Chemical energy:
-potential energy stored in chemical bonds
KEY CONCEPT:
-When energy is exchanged, heat is produced, but cells cannot capture it or use it for work
Break Down, Build Up
-Decomposition reaction (catabolism):
AB A +B
-Synthesis reaction (anabolism):
A+B AB
-Exchange reaction (reversible):
AB A+B
Water In, Water Out
-Hydrolysis
A-B-C-D-E+H20 A-B-C-H+HO-D-E
-Dehydration synthesis (condensation)
A-B-C-H+HO-D-E A-B-C-D-E+H2O
KEY CONCEPT:
-Reversible reactions seek equilibrium, balancing opposing reaction rates
-Add or remove reactants:
-reactions rates adjust to reach a new equilibrium
How do enzymes control metabolism?
Activation Energy
-Chemical reactions in cells cannot start without help
-Activation energy gets a reaction started
*PIC*
Material in Reactions
-Reactants
-materials going into a reaction
-Products:
-materials coming out of a reaction
-Enzymes:
-proteins that lower the activation energy of a reaction
Energy In, Energy Out
-Exergonic reactions:
-produce more energy than they use
-Endergonic reactions
-use more energy than they produce
KEY CONCEPT:
-Most chemical reactions that sustain life cannot occur unless the right enzymes are present
What is the difference between organic and inorganic compunds?
Organic and Inorganic Molecules
-Organic:
-molecules based on carbon and hydrogen
-Inorganic:
-molecules not based on carbon and hydrogen
Essential Molecules
-Nutrients
- essential molecules obtained from food
-Metabolites
-molecules made or broken down in the body
Why is water so important to life?
Properties of Water
-Solubility:
-water's ability to dissolve a solute in a solvent to make a solution
-reactivity:
-most body chemistry uses or occurs in water
-High heat capacity:
-water's ability to absorb and retain heat
-Lubrication:
- to moisten and reduce friction
KEY CONCEPT:
-most of our body wieght is water
-water is the key structural and functionsl component of cells and their control mechanisms, the nucliec acids
Aqueous Solutions
*PIC*
-Polar water molecules form hydration spheres around ions and small polar molecules to keep them in solution
Electrolytes
-inorganic ions which conduct electricity in solution
-electrolyte imbalance seriously disturbs vital body functions
*PIC*
Molecules and Water
-hydrophilic:
-hydro=water, philos=loving
-reacts with water
-hydrophobic:
-phobos= fear
- does not react with water
Solutions
-Colloid:
-a solution of very large organic molecules
-Suspension
-a solution in which particles settle (sediment)
-Concentration
-the amount of solute in a solvent (mol/L, mg/ML)
What is pH and why do we need buffers?
pH
-pH:
-the concentation of hydrogen ions (H+) in a solution
-Nuetral pH:
-a balance of H+ and OH-
-pure water= 7.0
Acids and Bases
-Acid (Acidic): pH lower than 7.0
-high H+ concentration,
low OH- concentration
-Base (basic): pH higher than 7.0
-low H+ concentration,
high OH- concentration
pH Scale
-Has an inverse relationship with H+ concentration:
-more H+ ions mean lower pH, less H+ ions mean higher pH
*PIC*
KEY CONCEPT:
-pH of body fluids measures free H+ ions in solution
-Excess H+ ions (low pH):
-damages cells and tissues
-alters proteins
-interferes with normal physiological functions
-Excess OH- ions (high pH) also cause problems, but rarely
Acid and Alkaline
-Acidosis:
-excess H+ in body fluid (low pH)
-Alkalosis:
-excess OH- in body fluid (high pH)
Controlling pH
-salts:
-positive or negative ions in solution
-contain no H+ or OH- (NaCl)
-buffers:
-weak aci/salt compunds
-neutralizes either strong acid or strong base
What kinds of organic compounds are there, and how do they work?
Functional Groups
-Molecular groups which allow molecules to interact with other molecules
*PIC*
Simple Sugars
*PIC*
Monosaccharides and Disaccharides
-Monosaccharides:
-simple sugars with 3 to 7 carbon atoms (glucose)
-Disaccharides:
- 2 simple sugars condensed by dehydration synthesis (sucrose)
Building and Breaking Down Sugars
Polysaccharides
-Chains of many simple sugars (glycogen)
*PIC*
Carbohydrate Functions
*PIC*
KEY CONCEPT
-carbohydrates are quick energy sources and components of membranes
-lipids have many functions, including membrane structure and energy storgae
Lipids
-mainly hydrophobic molecules such as fats, oils, and waxes
-made mostly of carbon and hydrogen atoms
Classes of Lipids:
-fatty acids
-eicosanoids
-glycerides
-steroids
-phospholipids and glycolipids
Fatty ACids
*PIC*
Saturated and Unsaturated
-fatty acids may be:
-saturated with hydrogen (no covalent bonds)
-unsaturated (1 or more double bonds)
Eicosanoids
*PIC*
Types of Eicosanoids
-Leukotrienes:
-active in immune system
-Prostaglandins
-local hormones, short-chain fatty acids
Glycerides
*PIC*
-Glycerides: are the fatty acids attached to a glycerol molecule
-Triglyceride: are the 3 fatty- acid tails, fat storage molecule
steroids
-4 carbon rings
*PIC*
types of steroids
-cholesterol
-component of cell membranes
-estrogens and testosterone:
-sex hormones
-corticosteroids and calcitrol:
-metabolic regulation
-bile salts
-derived from steroids
combination lipids
*PIC*
*PIC*
Phospholipids and Glycolipids
-have hydrophilic heads and hydrophobic tails
-are structural lipids, compnents of cell membranes
5 lipid types
*PIC*
Protein Structure
-Proteins are the most abundant and importnat organic molecules
-Basic elements:
- Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N)
-Basic building blocks:
-20 amino acids
Protein Functions
-7 major protein functions
-support
-structural proteins
-movement
-contractile proteins
-transport
-transport proteins
-buffering: regulation of pH
-metabolic regulation
-enzymes
-coordination and control:
-hormones
-defense:
-anitbodies
KEY CONCEPT:
-Proteins:
-control anatomical structure and physiological function
-determine cell shape and tissue properties
-perform almost all cell functions
AMino acids
*PIC*
AMino Acid Structure
1. central carbon
2. hydrogen
3. amino group (-NH2)
4. carboxylic acid group (-COOH)
5. variable side chain or R group
Peptides
*PIC*
Peptide Bond
-A dehydration synthesis between:
-the amino group of 1 amino acid
-and the carboxylic acid group of another amino acid
- producing a peptide
primary structure
-polypeptide:
-a long chain of amino acids
*PIC*
Secondary Structure
-hydrogen bonds from spirals or pleats
*PIC*
Tertiary Strucure
-Secondary structure folds into a unique shape
*PIC*
Quaternary Structure
-final protein shape:
-several tertiary structures together
*PIC*
Shape and Function
-Protein function is based on shape
-shape is based on sequence of amino acids
-Denaturation:
-loss of shape and function due to heat or pH
Protein Shapes
-fibrous proteins:
-structural sheets or strands
-Globular proteins:
-soluble spheres with active functions
Enzymes
-Enzymes are catalysts:
-proteins that lower the activation energy of a chemical reaction
- are not chnaged or used up in the reaction
How enzymes work
*PIC*
How enzymes work
-Substrates:
-reactants in enzymatic reactions
-ACtive site:
-a location on an enzyme that fits a particular substrate
Enzyme Helpers
-Cofactor:
-an ion or molecule that binds to an enzyme before substrates can bind
-Coenzyme:
-nonprotein organic cofactors (vitamins)
-Isozymes:
-2 enzymes that can catalyze the same reaction
Enzyme Characteristics
-Specificity:
-one enzyme catalyzes one reaction
-Saturation limits
- an enzyme's maximum work rate
-REgulation
-the ability to turn off and on
Protein COmbintations
Glycoproteins:
-large protein + small carbohydrate
- includes enzymes, antibodies, hormones, and mucus production
-Proteoglycans:
-large polysaccharides + polypeptides
-promote viscosity
Nucleic Acids:
-Large organic molecules found in the nucleus, which store and process information at the molecular level
-DNA and RNA
Deoxyribonucleic Acid (DNA)
-Determines inherited characteristics
-Directs protein synthesis
-controls enzymes production
-controls metabolism
Ribonucleic Acid (RNA)
-codes intermediate steps in protein synthesis
KEY CONCEPT:
-DNA in the cell nucleus contains the information need to construct all of the proteins in the body
Nucleotides
-Are the building blocks of DNA
-Have 3 molecular parts:
-sugar (deoxyribose)
-phosphate group
-nitrogenous base (A,G,T,C)
THe Bases
*PIC*
Complementary Bases
-Complementary Base Pairs:
-purines pair with pyrimidines:
-DNA:
-adenine (A) and thymine (T)
-cytosine (C) and guanine (G)
-RNA:
-uracil (U) replaces thymine (T)
Nucleic Acids
-Long chains of nucleotides form RNA and DNA
*PIC*
RNA and DNA
*PIC*
-RNA:
-a single strand
-DNA
-a double helix joined at bases by hydrogen bonds
Forms of RNA
-messenger RNA (mRNA)
-transfer RNA (tRNA)
-ribosomal RNA (rRNA)
ADP and ATP
-adenosine diphosphate (ADP):
-2 phosphate groups
-di=2
-adenosine triphosphate (ATP):
-3 phosphate groups
-tri=3
Phosphorylation
-adding a phosphate groups to ADP with a high-energy bond to form the high-energy compound ATP
-ATPase:
-the enzyme that catalyzes phophorylation
The Energy Molecule
-chemical energy stored in phosphate bonds
*PIC*
Compounds Important to Physiology
*PIC*
Recycling Old Molecules
*PIC*
KEY CONCEPT:
-your body recycles and renews all of its chemical components at intervals ranging from minutes to years.
-metabolic turnover lets your body grow, change and adapt to new condtions and acitivies.
SUMMARY:
-atoms, molecules, and chemical bonds control cellular physiology
-metabolism and energy work witht the cell
-importance of organic and inorganic nutrients and metabolites
- roles of water and solubility in metabolism and cell structure
-chemistry of acids and bases, ph and buffers
-structure and function of carbohydrates, lipds proteins, and nucleic acids.
How do atoms work?
KEY CONCEPT:
-Matter is made up of atoms
-Atoms join together to form chemicals with different characteristics
-Chemical characteristics determine physiology at the molecular and cellular level
The Atom
Atomic Particles
-Proton:
-positive, 1 mass unit
-Neutron:
-neutral, 1 mass unit
-Electron:
-negative, low mass
Particles and Mass
-Atomic Number:
-Number of protons
-Mass Number:
-Number of protons plus neutrons
-Atomic Weight:
-exact mass of all particles (daltons)
Isotopes
-2 or more elements with equal numbers of protons but different numbers of neutrons
Elements in the Human Body
*PIC*
-Are determined by the atomic number of an atom:
-are the most basic chemicals
Chemical Properties of Atoms
How do atoms forn molecules and compounds?
Chemical Bonds
-Ionic bonds:
-attraction between cations and anions
-Covalent bonds:
-strong electron bonds
-Hydrogen bonds:
-weak polar bonds
Molecules and Compounds:
-Molecules
-atoms joined by strong bonds
-Compunds
-atoms joined by strong or weak bonds
Ionic Bonds
*PIC*
-Are atoms with positive or negative charge
*PIC*
Covalent Bonds
Hydrogen Bonds
-Hydrogen bonds between H2O molecules cause surface tension
*PIC*
States of Matter
-Solid
-constand volume and shape
-Liquid
-constant volume but change shape
-Gas
-change volume and shape
Why are chemical reactions important to physiology?
Energy
-Energy
-the power to do work
-Work
-a change in mass or distance
Forms of Energy
-Kinetic energy
-energy of motion
-Potential Energy
-stored energy
-Chemical energy:
-potential energy stored in chemical bonds
KEY CONCEPT:
-When energy is exchanged, heat is produced, but cells cannot capture it or use it for work
Break Down, Build Up
-Decomposition reaction (catabolism):
AB A +B
-Synthesis reaction (anabolism):
A+B AB
-Exchange reaction (reversible):
AB A+B
Water In, Water Out
-Hydrolysis
A-B-C-D-E+H20 A-B-C-H+HO-D-E
-Dehydration synthesis (condensation)
A-B-C-H+HO-D-E A-B-C-D-E+H2O
KEY CONCEPT:
-Reversible reactions seek equilibrium, balancing opposing reaction rates
-Add or remove reactants:
-reactions rates adjust to reach a new equilibrium
How do enzymes control metabolism?
Activation Energy
-Chemical reactions in cells cannot start without help
-Activation energy gets a reaction started
*PIC*
Material in Reactions
-Reactants
-materials going into a reaction
-Products:
-materials coming out of a reaction
-Enzymes:
-proteins that lower the activation energy of a reaction
Energy In, Energy Out
-Exergonic reactions:
-produce more energy than they use
-Endergonic reactions
-use more energy than they produce
KEY CONCEPT:
-Most chemical reactions that sustain life cannot occur unless the right enzymes are present
What is the difference between organic and inorganic compunds?
Organic and Inorganic Molecules
-Organic:
-molecules based on carbon and hydrogen
-Inorganic:
-molecules not based on carbon and hydrogen
Essential Molecules
-Nutrients
- essential molecules obtained from food
-Metabolites
-molecules made or broken down in the body
Why is water so important to life?
Properties of Water
-Solubility:
-water's ability to dissolve a solute in a solvent to make a solution
-reactivity:
-most body chemistry uses or occurs in water
-High heat capacity:
-water's ability to absorb and retain heat
-Lubrication:
- to moisten and reduce friction
KEY CONCEPT:
-most of our body wieght is water
-water is the key structural and functionsl component of cells and their control mechanisms, the nucliec acids
Aqueous Solutions
*PIC*
-Polar water molecules form hydration spheres around ions and small polar molecules to keep them in solution
Electrolytes
-inorganic ions which conduct electricity in solution
-electrolyte imbalance seriously disturbs vital body functions
*PIC*
Molecules and Water
-hydrophilic:
-hydro=water, philos=loving
-reacts with water
-hydrophobic:
-phobos= fear
- does not react with water
Solutions
-Colloid:
-a solution of very large organic molecules
-Suspension
-a solution in which particles settle (sediment)
-Concentration
-the amount of solute in a solvent (mol/L, mg/ML)
What is pH and why do we need buffers?
pH
-pH:
-the concentation of hydrogen ions (H+) in a solution
-Nuetral pH:
-a balance of H+ and OH-
-pure water= 7.0
Acids and Bases
-Acid (Acidic): pH lower than 7.0
-high H+ concentration,
low OH- concentration
-Base (basic): pH higher than 7.0
-low H+ concentration,
high OH- concentration
pH Scale
-Has an inverse relationship with H+ concentration:
-more H+ ions mean lower pH, less H+ ions mean higher pH
*PIC*
KEY CONCEPT:
-pH of body fluids measures free H+ ions in solution
-Excess H+ ions (low pH):
-damages cells and tissues
-alters proteins
-interferes with normal physiological functions
-Excess OH- ions (high pH) also cause problems, but rarely
Acid and Alkaline
-Acidosis:
-excess H+ in body fluid (low pH)
-Alkalosis:
-excess OH- in body fluid (high pH)
Controlling pH
-salts:
-positive or negative ions in solution
-contain no H+ or OH- (NaCl)
-buffers:
-weak aci/salt compunds
-neutralizes either strong acid or strong base
What kinds of organic compounds are there, and how do they work?
Functional Groups
-Molecular groups which allow molecules to interact with other molecules
*PIC*
Simple Sugars
*PIC*
Monosaccharides and Disaccharides
-Monosaccharides:
-simple sugars with 3 to 7 carbon atoms (glucose)
-Disaccharides:
- 2 simple sugars condensed by dehydration synthesis (sucrose)
Building and Breaking Down Sugars
Polysaccharides
-Chains of many simple sugars (glycogen)
*PIC*
Carbohydrate Functions
*PIC*
KEY CONCEPT
-carbohydrates are quick energy sources and components of membranes
-lipids have many functions, including membrane structure and energy storgae
Lipids
-mainly hydrophobic molecules such as fats, oils, and waxes
-made mostly of carbon and hydrogen atoms
Classes of Lipids:
-fatty acids
-eicosanoids
-glycerides
-steroids
-phospholipids and glycolipids
Fatty ACids
*PIC*
Saturated and Unsaturated
-fatty acids may be:
-saturated with hydrogen (no covalent bonds)
-unsaturated (1 or more double bonds)
Eicosanoids
*PIC*
Types of Eicosanoids
-Leukotrienes:
-active in immune system
-Prostaglandins
-local hormones, short-chain fatty acids
Glycerides
*PIC*
-Glycerides: are the fatty acids attached to a glycerol molecule
-Triglyceride: are the 3 fatty- acid tails, fat storage molecule
steroids
-4 carbon rings
*PIC*
types of steroids
-cholesterol
-component of cell membranes
-estrogens and testosterone:
-sex hormones
-corticosteroids and calcitrol:
-metabolic regulation
-bile salts
-derived from steroids
combination lipids
*PIC*
*PIC*
Phospholipids and Glycolipids
-have hydrophilic heads and hydrophobic tails
-are structural lipids, compnents of cell membranes
5 lipid types
*PIC*
Protein Structure
-Proteins are the most abundant and importnat organic molecules
-Basic elements:
- Carbon (C), hydrogen (H), oxygen (O), and nitrogen (N)
-Basic building blocks:
-20 amino acids
Protein Functions
-7 major protein functions
-support
-structural proteins
-movement
-contractile proteins
-transport
-transport proteins
-buffering: regulation of pH
-metabolic regulation
-enzymes
-coordination and control:
-hormones
-defense:
-anitbodies
KEY CONCEPT:
-Proteins:
-control anatomical structure and physiological function
-determine cell shape and tissue properties
-perform almost all cell functions
AMino acids
*PIC*
AMino Acid Structure
1. central carbon
2. hydrogen
3. amino group (-NH2)
4. carboxylic acid group (-COOH)
5. variable side chain or R group
Peptides
*PIC*
Peptide Bond
-A dehydration synthesis between:
-the amino group of 1 amino acid
-and the carboxylic acid group of another amino acid
- producing a peptide
primary structure
-polypeptide:
-a long chain of amino acids
*PIC*
Secondary Structure
-hydrogen bonds from spirals or pleats
*PIC*
Tertiary Strucure
-Secondary structure folds into a unique shape
*PIC*
Quaternary Structure
-final protein shape:
-several tertiary structures together
*PIC*
Shape and Function
-Protein function is based on shape
-shape is based on sequence of amino acids
-Denaturation:
-loss of shape and function due to heat or pH
Protein Shapes
-fibrous proteins:
-structural sheets or strands
-Globular proteins:
-soluble spheres with active functions
Enzymes
-Enzymes are catalysts:
-proteins that lower the activation energy of a chemical reaction
- are not chnaged or used up in the reaction
How enzymes work
*PIC*
How enzymes work
-Substrates:
-reactants in enzymatic reactions
-ACtive site:
-a location on an enzyme that fits a particular substrate
Enzyme Helpers
-Cofactor:
-an ion or molecule that binds to an enzyme before substrates can bind
-Coenzyme:
-nonprotein organic cofactors (vitamins)
-Isozymes:
-2 enzymes that can catalyze the same reaction
Enzyme Characteristics
-Specificity:
-one enzyme catalyzes one reaction
-Saturation limits
- an enzyme's maximum work rate
-REgulation
-the ability to turn off and on
Protein COmbintations
Glycoproteins:
-large protein + small carbohydrate
- includes enzymes, antibodies, hormones, and mucus production
-Proteoglycans:
-large polysaccharides + polypeptides
-promote viscosity
Nucleic Acids:
-Large organic molecules found in the nucleus, which store and process information at the molecular level
-DNA and RNA
Deoxyribonucleic Acid (DNA)
-Determines inherited characteristics
-Directs protein synthesis
-controls enzymes production
-controls metabolism
Ribonucleic Acid (RNA)
-codes intermediate steps in protein synthesis
KEY CONCEPT:
-DNA in the cell nucleus contains the information need to construct all of the proteins in the body
Nucleotides
-Are the building blocks of DNA
-Have 3 molecular parts:
-sugar (deoxyribose)
-phosphate group
-nitrogenous base (A,G,T,C)
THe Bases
*PIC*
Complementary Bases
-Complementary Base Pairs:
-purines pair with pyrimidines:
-DNA:
-adenine (A) and thymine (T)
-cytosine (C) and guanine (G)
-RNA:
-uracil (U) replaces thymine (T)
Nucleic Acids
-Long chains of nucleotides form RNA and DNA
*PIC*
RNA and DNA
*PIC*
-RNA:
-a single strand
-DNA
-a double helix joined at bases by hydrogen bonds
Forms of RNA
-messenger RNA (mRNA)
-transfer RNA (tRNA)
-ribosomal RNA (rRNA)
ADP and ATP
-adenosine diphosphate (ADP):
-2 phosphate groups
-di=2
-adenosine triphosphate (ATP):
-3 phosphate groups
-tri=3
Phosphorylation
-adding a phosphate groups to ADP with a high-energy bond to form the high-energy compound ATP
-ATPase:
-the enzyme that catalyzes phophorylation
The Energy Molecule
-chemical energy stored in phosphate bonds
*PIC*
Compounds Important to Physiology
*PIC*
Recycling Old Molecules
*PIC*
KEY CONCEPT:
-your body recycles and renews all of its chemical components at intervals ranging from minutes to years.
-metabolic turnover lets your body grow, change and adapt to new condtions and acitivies.
SUMMARY:
-atoms, molecules, and chemical bonds control cellular physiology
-metabolism and energy work witht the cell
-importance of organic and inorganic nutrients and metabolites
- roles of water and solubility in metabolism and cell structure
-chemistry of acids and bases, ph and buffers
-structure and function of carbohydrates, lipds proteins, and nucleic acids.
Chapter 1 Notes
Chapter One: An Introduction to Anatomy and Physiology
What is Anatomy and Physiology?
Anatomy:
-Describes the structures of the body
-What they are made of
-Where they are located
-Associated structures
Physiology:
-Is the study of:
-Functions of anatomical structures
-Individual and cooperative functions
KEY CONCEPT:
-All physiological functions are performed by specific anatomical structures
-These functions follow standard physical and mechanical principles
What do anatomists and physiologists do?
Specialties of Anatomy:
-Gross Anatomy, or macroscopic anatomy examines large, visible structures:
-Surface anatomy:
-Exterior features
-Regional anatomy:
-Body areas
-Systemic anatomy:
-Groups of organs working together
-Developmental anatomy:
-From egg (embryology) to maturity
-Clinical anatomy:
-Medical Specialties
-Microscopic anatomy examines cells and molecules:
-Cytology:
-Cells and their structures
-Cyt=cell
-Histology:
-Tissues and their structures
Specialties of Physiology
-Cell physiology
-Processes within and between cells
-Special physiology
-Functions of specific organs
-Systemic physiology:
-Functions of an organ system
-Pathological physiology:
-Effects of diseases
How are living things organized?
From Simple to Complex
-Atoms
-Are the smalled chemical units
-Molecules
-Are a group of atoms working together
-Organelles:
-Are a group of molecules working together
-Cells:
-Are a group of organelles working together
-Tissues:
-Are a group of similar cells working together
-Organs:
Are a group of different tissues working together
-Organ System:
-Are a group of organs working together
-Organism:
-is an individual
Organizing a Muscle
-Protein molecules (chemical level)
-Protein filaments (organelle level)
-Muscle cells (cellular level)
-Cardiac muscle tissue (tissue level)
-Heart (organ level)
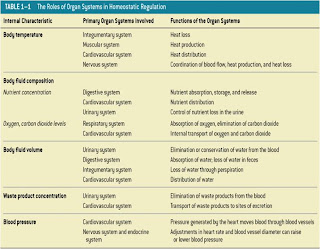
The 11 Organ Systems
KEY CONCEPT:
-The body is divided into 11 organ systems
-All organ systems work together
-Many organs work in more than 1 system
-Homeostasis: All body systems working together to maintain a stable internal environment.
-Systems respond to external and internal changes to functions within a normal range (body temperature, fluid balance)
-Failure to function within a normal range results in disease
Mechanisms of Regulation
-Autoregulation (intrinsic):
-Automatic response in a cell, tissue, or organ
-Extrinsic regulation:
-Responses controlled by nervous and endocrine systems
Maintaining Normal Limits
Maintaining Normal Limits
-Receptor:
-Recieves the stimulus
-Control center:
-Processes the signal and sends instructions
-Effector:
-Carries out instructions
Negative Feedback
-The response of the effector negates the stimulus
Positive Feedback
-The responseof the effector reinforces the stimulus
Working Together
-Systems integration:
-Systems work together to maintain homeostasis
KEY CONCEPT:
-Homeostasis is a state of equilibrium:
-opposing forces are in balance
-Physiological systems work to restore balance
-Failure results in disease or death
What are the anatomical terms used to describe body sections, regions, and relative positions?
Anatomical Landmarks
-Superficial characteristics:
-Surface parts
-Names
-Adjectives
KEY CONCEPT:
-Anatomical position:
-Hands at sides, palms forward
-Supine:
-Lying down, face up
-Prone:
-Lying down, face down
Quadrants and Regions
- 4 abdominopelvic quadrants around umbilicus
-9 abdominopelvic regions
-Internal organs associated with abdominopelvic regions
Which Direction?
-Lateral:
-Side view
-Frontal:
-Front view
-Anatomical Direction:
-Refers to the patient's left or right
3 Dimensions
Plane:
-A 3 dimensional axis
Section:
-A slice parallel to a plane
What are the major body cavities and their subdivisons?
The Ventral Body Cavity
- Coelom:
-Divided by the diaphragm into the thoracic cavity and the abdominopelivc cavity
Isolating the Organs
-Serous membranes:
-Consist of perietal layer and visceral layer
Dividing the Cavities
-Thoracic Cavity:
-Divided the mediastinum into 2 pleural cavities
SUMMARY:
-Structure and function in anatomy and physiology
-Vocabulary and anatomical terms
-Levels of physical organization
-Homeostasis and feedback
-Systems integration and equilibrium
-Dividing and describing the body
-Locations and functions of major organ systems
What is Anatomy and Physiology?
Anatomy:
-Describes the structures of the body
-What they are made of
-Where they are located
-Associated structures
Physiology:
-Is the study of:
-Functions of anatomical structures
-Individual and cooperative functions
KEY CONCEPT:
-All physiological functions are performed by specific anatomical structures
-These functions follow standard physical and mechanical principles
What do anatomists and physiologists do?
Specialties of Anatomy:
-Gross Anatomy, or macroscopic anatomy examines large, visible structures:
-Surface anatomy:
-Exterior features
-Regional anatomy:
-Body areas
-Systemic anatomy:
-Groups of organs working together
-Developmental anatomy:
-From egg (embryology) to maturity
-Clinical anatomy:
-Medical Specialties
-Microscopic anatomy examines cells and molecules:
-Cytology:
-Cells and their structures
-Cyt=cell
-Histology:
-Tissues and their structures
Specialties of Physiology
-Cell physiology
-Processes within and between cells
-Special physiology
-Functions of specific organs
-Systemic physiology:
-Functions of an organ system
-Pathological physiology:
-Effects of diseases
How are living things organized?
From Simple to Complex
-Atoms
-Are the smalled chemical units
-Molecules
-Are a group of atoms working together
-Organelles:
-Are a group of molecules working together
-Cells:
-Are a group of organelles working together
-Tissues:
-Are a group of similar cells working together
-Organs:
Are a group of different tissues working together
-Organ System:
-Are a group of organs working together
-Organism:
-is an individual
Organizing a Muscle
-Protein molecules (chemical level)
-Protein filaments (organelle level)
-Muscle cells (cellular level)
-Cardiac muscle tissue (tissue level)
-Heart (organ level)
The 11 Organ Systems
KEY CONCEPT:
-The body is divided into 11 organ systems
-All organ systems work together
-Many organs work in more than 1 system
-Homeostasis: All body systems working together to maintain a stable internal environment.
-Systems respond to external and internal changes to functions within a normal range (body temperature, fluid balance)
-Failure to function within a normal range results in disease
Mechanisms of Regulation
-Autoregulation (intrinsic):
-Automatic response in a cell, tissue, or organ
-Extrinsic regulation:
-Responses controlled by nervous and endocrine systems
Maintaining Normal Limits
Maintaining Normal Limits
-Receptor:
-Recieves the stimulus
-Control center:
-Processes the signal and sends instructions
-Effector:
-Carries out instructions
Negative Feedback
-The response of the effector negates the stimulus
Positive Feedback
-The responseof the effector reinforces the stimulus
Working Together
-Systems integration:
-Systems work together to maintain homeostasis
KEY CONCEPT:
-Homeostasis is a state of equilibrium:
-opposing forces are in balance
-Physiological systems work to restore balance
-Failure results in disease or death
What are the anatomical terms used to describe body sections, regions, and relative positions?
Anatomical Landmarks
-Superficial characteristics:
-Surface parts
-Names
-Adjectives
KEY CONCEPT:
-Anatomical position:
-Hands at sides, palms forward
-Supine:
-Lying down, face up
-Prone:
-Lying down, face down
Quadrants and Regions
- 4 abdominopelvic quadrants around umbilicus
-9 abdominopelvic regions
-Internal organs associated with abdominopelvic regions
Which Direction?
-Lateral:
-Side view
-Frontal:
-Front view
-Anatomical Direction:
-Refers to the patient's left or right
3 Dimensions
-A 3 dimensional axis
Section:
-A slice parallel to a plane
What are the major body cavities and their subdivisons?
The Ventral Body Cavity
- Coelom:
-Divided by the diaphragm into the thoracic cavity and the abdominopelivc cavity
Isolating the Organs
-Serous membranes:
-Consist of perietal layer and visceral layer
Dividing the Cavities
-Thoracic Cavity:
-Divided the mediastinum into 2 pleural cavities
SUMMARY:
-Structure and function in anatomy and physiology
-Vocabulary and anatomical terms
-Levels of physical organization
-Homeostasis and feedback
-Systems integration and equilibrium
-Dividing and describing the body
-Locations and functions of major organ systems
Subscribe to:
Comments (Atom)